Chemical Engineering :: Chemical Reaction Engineering
-
The conversion for a first order liquid phase reaction. A
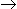 B in a CSTR is 50%. If another CSTR of the same volume is connected in series, then the % conversion at the exit of the second reactor will be
B in a CSTR is 50%. If another CSTR of the same volume is connected in series, then the % conversion at the exit of the second reactor will be -
Rate of a chemical reaction is independent of the concentration of the reactants for a __________ reaction.
-
With increase in temperature, the rate constant obeying Arhenious equation
-
Bulk diffusion in catalyst pore __________ with increase in pressure.
-
Pick out the correct statement.
-
The concentration of A in a first order reaction, A
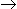 B, decreases
B, decreases -
The equilibrium constant of chemical reaction __________ in the presence of catalyst.
-
The order of a chemical reaction is
-
A chemical reaction occurs, when the energy of the reacting molecules is __________ the activation energy of reaction.
-
In the hydrodealkylation of toluene to benzene, the following reactions occur:
C7H8 + H2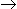 C6H6 + CH4
C6H6 + CH4
2C6H6 C12H10 + H2
C12H10 + H2
Toluene and hydrogen are fed to a reactor in a molar ratio 1:5.80% of the toluene gets converted and the selectivity of benzene(defined as moles of benzene formed/moles of toluene converted) is 90%. The fractional conversion of hydrogen is
|
A.
A chemical reaction accompanied by absorption of heat is called an exothermic reaction.
|
|
B.
A chemical reaction accompanied by evolution of heat is called an endother-mic reaction.
|
|
C.
The rate constant for a first order reaction does not change on changing the concentration units.
|
|
D.
Chemical equilibrium state is dynamic in nature.
|


 Whatsapp
Whatsapp
 Facebook
Facebook

