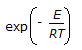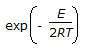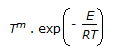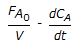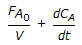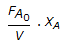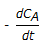Chemical Engineering :: Chemical Reaction Engineering
-
The minimum energy required to allow a chemical reaction to proceed is termed as the 'threshold energy '. Chemical reaction with low activation energy are
-
Catalytic action in a catalytic chemical reaction follows from the ability of catalyst to change the
-
In Langmuir treatment of adsorption,
-
Organic catalysts differ from the inorganic catalyst in the sense that the former is
-
An endothermic aqueous phase first order irreversible reaction is carried out in an adiabatic plug flow reactor. The rate of reaction
-
Pick out the wrong statement.
-
An endothermic second order reaction is carried out in an adiabatic plug flow reactor. The rate of heat generation is
-
Equilibrium of a chemical reaction as viewed by kinetics is a __________ state.
|
A.
whole surface of the catalyst does not have the same activity for adsorption and there is attraction between the adsorbed molecule.
|
|
B.
whole surface of the catalyst is essentially uniform and the adsorbed molecule has no effect on the rate of adsorption per site.
|
|
C.
all the adsorption does not take place by the same mechanism.
|
|
D.
extent of adsorption is more than one complete monomolecular layer on the surface.
|
|
A.
Exit age description function (E) and internal age distribution function (I) are related as,
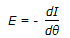 . . |
|
B.
Chmisorption studies are useful in the determination of catalyst surface area and pore size distribution.
|
|
C.
A higher temperature favours the reaction of higher activation energy.
|
|
D.
A catalyst increases the potential energy barrier over which the reactants must pass to form products.
|


 Whatsapp
Whatsapp
 Facebook
Facebook