Chemical Engineering :: Chemical Reaction Engineering
-
Which of the following will give maximum gas conversion ?
-
__________ explains the mechanism of catalysis.
-
From among the following, choose one which is not an exothermic process.
-
The fractional volume change of the system for the isothermal gas phase reaction, A
 3B , between no conversion and complete conversion is
3B , between no conversion and complete conversion is -
A catalyst
-
What is the order of a chemical reaction,
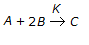 , if the rate of formation of 'C', increases by a factor of 2.82 on doubling the concentration of 'A' and increases by a factor of 9 on trebling the concentration of 'B'?
, if the rate of formation of 'C', increases by a factor of 2.82 on doubling the concentration of 'A' and increases by a factor of 9 on trebling the concentration of 'B'? -
For high conversion in a highly exothermic solid catalysed reaction, use a __________ bed reactor.
-
For every 10°C rise in temperature, the rate of chemical reaction doubles. When the temperature is increased from 30 to 70°C, the rate of reaction increases __________ times.
-
The single parameter model proposed for describing non-ideal flow is the __________ model.
-
A first order reaction requires two equal sized CSTR. The conversion is


 Whatsapp
Whatsapp
 Facebook
Facebook

