Chemical Engineering :: Chemical Reaction Engineering
-
An isothermal aqueous phase reversible reaction, P
 R, is to be carried out in a mixed flow reactor. The reaction rate in k.mole/m3 .h is given by, r = 0.5CP - 0.125CR. A stream containing only P enters the reactor. The residence time required (in hours) for 40% conversion of P is
R, is to be carried out in a mixed flow reactor. The reaction rate in k.mole/m3 .h is given by, r = 0.5CP - 0.125CR. A stream containing only P enters the reactor. The residence time required (in hours) for 40% conversion of P is -
Design of heterogeous catalytic reactor involves consideration of __________ steps.
-
A consecutive reaction,
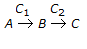 , is characterised by
, is characterised by -
What is the unit of the rate constant in a chemical reaction in which 10% of the reactant decomposes in one hour, 20% in two hours, 30% in three hours and so on ?
-
For a fluidised bed reactor, the most suitable/relevant model is a __________ model.
-
The rate at which a chemical substance reacts is proportional to its
-
In a reaction, the threshold energy is equal to (where, A = activation energy N = normal energy of reactants)
-
Thermodynamic equilibrium constant in a system is affected by
-
The most suitable reactor for carrying out an auto-thermal reaction is a
-
Which of the following chemical reactions will be favoured by low pressure ?


 Whatsapp
Whatsapp
 Facebook
Facebook

