Chemical Engineering :: Chemical Reaction Engineering
-
Second order consecutive irreversible reactions
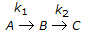 were carried out in a constant volume isothermal batch reactor with different initial feed compositions. Reactor temperature was same in all the cases. In experiments where the ratio of concentration of B to that of A in the initial feed was less than 0.5, the concentration of B increased first, reached a maximum and then declined with time. However, for all experiments where this concentration ratio was 0.5 or above, concentration of B decreased monotonically with time right from the beginning. What is the ratio of the two rate constants (k1/k2)?
were carried out in a constant volume isothermal batch reactor with different initial feed compositions. Reactor temperature was same in all the cases. In experiments where the ratio of concentration of B to that of A in the initial feed was less than 0.5, the concentration of B increased first, reached a maximum and then declined with time. However, for all experiments where this concentration ratio was 0.5 or above, concentration of B decreased monotonically with time right from the beginning. What is the ratio of the two rate constants (k1/k2)? -
Pick out the wrong statement.
-
There is no correspondence between stoichiometry and the rate equation in case of a/an __________ reaction.
-
A spherical porous catalyst particle of radius R is subjected to reactant A which reacts to form B by a zero order surface reaction A
 B. Film mass transfer resistance is negligible and pore diffusion of A is rate controlling. The effectiveness factor of the catalyst is reported as 7/8. Which of the following statement is true?
B. Film mass transfer resistance is negligible and pore diffusion of A is rate controlling. The effectiveness factor of the catalyst is reported as 7/8. Which of the following statement is true? -
Which of the following is used for calcination of limestone and dolomite in industrial practice ?
-
An imbalanced chemical reaction equation is against the law of
-
Which of the following is a characteristic of an ideal plug flow reactor ?
-
Velocity of a reaction depends upon the
-
Threshold energy in a reaction is equal to the
|
A.
Catalytic activity of enzyme catalysed reactions which is affected by temperature, pH value & chemical agents, is maximum at a temperature of about 45°C.
|
|
B.
Most of the enzyme catalysed reactions involve at least two substrates.
|
|
C.
Enzymes help in increasing the activation energy of the reaction.
|
|
D.
Equilibrium concentrations in enzyme catalysed reactions can be calculated by using the thermodynamic properties of substrates & products.
|
|
A.
Inner catalyst core of radius R/8 does not participate in reaction.
|
|
B.
Inner catalyst core of radius R/2 does not participate in reaction.
|
|
C.
Inner catalyst core of radius 7R/8 does not participate in reaction.
|
|
D.
Effectiveness factor for a zero order reaction can not be 7/8 as it must always be 1.
|


 Whatsapp
Whatsapp
 Facebook
Facebook

