Chemical Engineering :: Chemical Reaction Engineering
-
The conversion for a second order, irreversible reaction (constant volume),
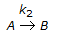 , in batch mode is given by
, in batch mode is given by -
Pick out the wrong statement.
-
A backmix reactor
-
Rate of a chemical reaction is not influenced by the
-
Pick out the wrong statement:
-
The reaction A
 B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature))
B is conducted in an adiabatic plug flow reactor (PFR). Pure A at a concentration of 2 kmol/m3 is fed to the reactor at the rate of 0.01 m3 /s and at a temperature of 500 K. If the exit conversion is 20%, then the exit temperature (in k)is (Data: Heat of reaction at 298 K = - 50000 kJ/ kmole of A reacted Heat capacities CPA = CPB = 100kJ/kmole. K (may be assumed to be independent of temperature)) -
Which of the following is not a theory of homogeneous reaction?
-
A first order homogeneous reaction of the type X
 Y
Y  Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time?
Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the concentration of X, Y and Z with time? 
-
In a semi-batch reactor,
-
Most important characteristics of gas-liquid reactors are the
|
A.
The integral method of analysing kinetic data is used when the data is scattered.
|
|
B.
The differential method of analysing kinetic data requires more accurate or larger amounts of data.
|
|
C.
When the reaction rate is independent of temperature, the reaction is said to be of zero order.
|
|
D.
The ratio of volumes of plug flow reactor to that of mixed reactor is always less than one for identical feed composition, flow rate, conversion and for all positive reaction orders.
|
|
A.
Chemical reactions with high activation energy are very temperature sensitive.
|
|
B.
A flat velocity profile exists in a plug flow reactor.
|
|
C.
The residence time for all the elements of fluid in case of a P.F.R. need not be same.
|
|
D.
Half life of a reaction increases with increased initial concentration for reaction orders more than one.
|






 Whatsapp
Whatsapp
 Facebook
Facebook

