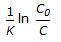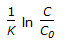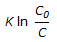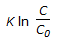Chemical Engineering :: Chemical Reaction Engineering
-
Photochemical reaction rate does not depend significantly on temperature, because
-
A CSTR is to be designed in which an exothermic liquid phase first order reaction of the type, A
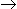 R, is taking place. The reactor is to be provided with a jacket in which coolant is flowing. Following data is given: CA0= 5 kmole/m3 ; XA = 0.5; Feed temperature = reactor temperature = 40°C. Rate constant at 40°C = 1 min-1 ; (ΔH) = - 40kJ/mole; ρ = 1000kg/m3 CP = 4 J/gm.°C ; q = 10-3 m3/min (ρ and CP are same for the reactant and product streams). The amount of heat to be removed is
R, is taking place. The reactor is to be provided with a jacket in which coolant is flowing. Following data is given: CA0= 5 kmole/m3 ; XA = 0.5; Feed temperature = reactor temperature = 40°C. Rate constant at 40°C = 1 min-1 ; (ΔH) = - 40kJ/mole; ρ = 1000kg/m3 CP = 4 J/gm.°C ; q = 10-3 m3/min (ρ and CP are same for the reactant and product streams). The amount of heat to be removed is -
According to the 'law of mass action', the rate of reaction is directly proportional to the
-
For an ideal gas mixture undergoing a reversible gaseous phase chemical reaction, the equilibrium constant
-
In the reversible reaction of the type, A + B
 AB, in general
AB, in general -
A batch reactor is
-
For an isothermal second order aqueous phase reaction, A
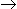 B, the ratio of the time required for 90% conversion to the time required for 45% conversion is
B, the ratio of the time required for 90% conversion to the time required for 45% conversion is -
A chemical reaction is of zero order, when the reaction rate is (where, CA = concentration of reactant)
|
A.
both forward and backward reactions will be exothermic.
|
|
B.
neither of the reactions will be en-dothermic.
|
|
C.
the combination reaction will be exothermic, while the dissociation reaction will be endothermic.
|
|
D.
the combination reaction will be endothermic, while the dissociation reaction will be exothermic.
|


 Whatsapp
Whatsapp
 Facebook
Facebook