Chemical Engineering :: Chemical Reaction Engineering
-
The exit age distribution of fluid leaving a vessel is used to know the
-
From Arhenius law, a plot of loge K versus 1/T gives a straight line with a slope of (-E/R). The unit of E/R is
-
In the converter of the contact process for the manufacture of H2SO4 the equilibrium conversion of SO2 __________ (A) __________ with increase in temperature and __________ (B) __________ with increase in the mole ratio of SO2 to air.
-
For the irreversible elementary first order reaction in parallel viz.
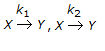 , a plot of Cy Vs. Cz will give a straight line having a slope of
, a plot of Cy Vs. Cz will give a straight line having a slope of -
The time needed to achieve the same fractional conversion for particles of different sizes (d) when chemical reaction controls, is proportional to
-
A balanced chemical reaction equation conforms to the law of
-
An exothermic reaction takes place in an adiabatic reactor. The product temperature __________ reactor feed temperature.
-
Reverse reaction in a chemical equilibrium is favoured by the
-
The equilibrium constant for the reversible reaction,
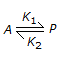 , is affected by the
, is affected by the -
Sum of the powers of the concentration terms in the rate equation is called the __________ of the reaction.


 Whatsapp
Whatsapp
 Facebook
Facebook

