Chemical Engineering :: Chemical Reaction Engineering
-
If helium is introduced in a reactor containing O2, SO2 and SO3 at equilibrium, so that total pressure increases while volume and temperature remains constant. In this case the dissociation of SO3 (as per Le Chatlier principle)
-
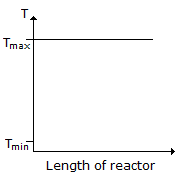
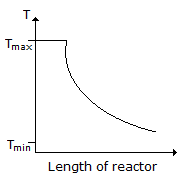
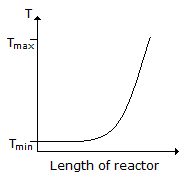
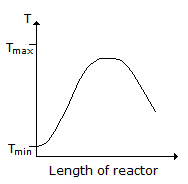
Consider a reversible exothermic reaction in a plug flow reactor. The maximum and minimum permissible temperatures are Tmax and Tmin respectively. Which of the following temperature (T) profiles will require the shortest residence time to achieve the desired conversion.
-
Fluidised bed reactor is characterised by
-
In which of the following gaseous phase reversible reactions, the product yield can not be increased by the application of high pressure ?
-
Space time equals the mean residence time
-
A liquid decomposes by irreversible first order kinetics and the half life period of this reaction is 8 minutes. The time required for 75% conversion of the liquid will be __________ minutes.
-
Rate constant 'k' and absolute temperature 'T' are related by collision theory (for bimolecular) as


 Whatsapp
Whatsapp
 Facebook
Facebook


 2NO
2NO