Chemical Engineering :: Chemical Reaction Engineering
-
Pure A in gas phase enters a reactor 50% of this A is converted to B through the reaction, A
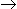 3B. Mole fraction of A in the exit stream is
3B. Mole fraction of A in the exit stream is -
The rate of reaction does not decrease appreciably as the reaction proceeds in case of __________ reactions.
-
For all positive reaction orders for a particular duty,
-
The heat of reaction
-
Which of the following does not produce a change in the value of rate constant of a reaction ?
-
With increase in temperature, the equilibrium conversion of a reversible exothermic reaction
-
In a CSTR __________ varies with time.
-
The following gas phase reactions are carried out isothermally in a CSTR.
A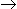 2R ; r1 = k1pA ;
2R ; r1 = k1pA ;
k1 = 20mole/(sec.m3 bar)
A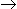 3S ; r2 = k2 pA ;
3S ; r2 = k2 pA ;
k2 = 40mole/(sec.m3 .bar)
What is the maximum possible value of FR(mole/sec.) ? -
As the chemical reaction proceeds, the rate of reaction
-
Pick out the wrong statement.


 Whatsapp
Whatsapp
 Facebook
Facebook

