Chemical Engineering :: Chemical Engineering Thermodynamics
-
When pressure is applied on the system, ice
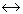 water, then
water, then -
Partial molar free energy of an element A in solution is same as its
-
Heat pump
-
The temperature at which both liquid and gas phases are identical, is called the __________ point.
-
What is the value of ln y (where y = activity co-efficient) for ideal gases ?
-
A closed system is cooled reversibly from 100°C to 50°C. If no work is done on the system
-
The partial pressure of each constituent present in an alloy is __________ the total vapor pressure exerted by the alloy.
-
Heat is added at constant pressure in an ideal __________ cycle.


 Whatsapp
Whatsapp
 Facebook
Facebook

