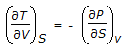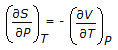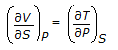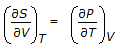Chemical Engineering :: Chemical Engineering Thermodynamics
-
Refrigeration capacity of a household refrigerator may be round about __________ tons.
-
An ideal gas is taken around the cycle ABCA as shown in P-V diagram below :
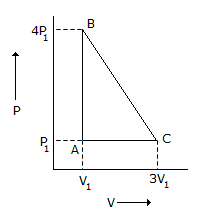
The work done by the gas during the cycle is equal to -
Chemical potential is a/an
-
In polytropic process (PVn = constant), if n = 1; it means a/an __________ process.
-
The melting point of paraffin wax (which contracts on solidification) __________ with pressure rise.
-
Co-efficient of Performance (COP) of a refrigerator is the ratio of the
-
The Maxwell relation derived from the differential expression for the Helmholtz free energy (dA) is
-
dW and dq are not the exact differential, because q and W are


 Whatsapp
Whatsapp
 Facebook
Facebook


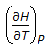 is the mathematical expression for
is the mathematical expression for