Chemical Engineering :: Chemical Engineering Thermodynamics
-
Mollier diagram is a plot of
-
The activity of an ideal gas is numerically __________ its pressure.
-
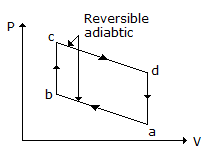
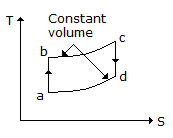
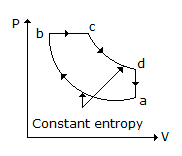
Specific heat of a gas for a reversible adiabatic process is
-
When a gas in a vessel expands, its internal energy decreases. The process involved is
-
A system is said to be isopiestic, if there is no __________ change.
-
In a turbine, the fluid expands almost
-
Specific/molar Gibbs free energy for a pure substance does not change during


 Whatsapp
Whatsapp
 Facebook
Facebook


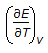 is the mathematical expression for
is the mathematical expression for