GATE 2017-2018 :: GATE Chemistry
- I n the proton decoupled 13C NMR spectrum of 7-norbornanone, the number of signals obtained is
- If Δy and Δpy are the uncertainties in the y-coordinate and the y component of the momentum of a particle respectively, then, according to uncertainty principle ΔyΔpy is (ћ = h/2π and h is Planck's constant)
- The average length of a typical α-helix comprised of 10 amino acids is
- Number of thymine residues in a 5000 kb DNA containing 23% guanine residues is
-
Shown below is a Hammett plot obtained for the reaction
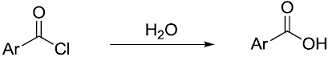
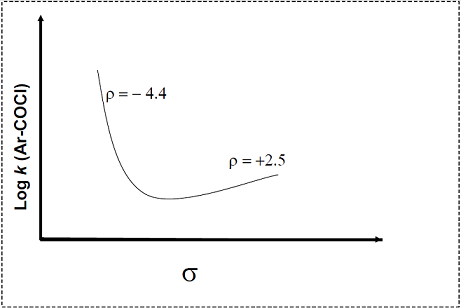 The change in slope of the plot indicates that
The change in slope of the plot indicates that - T he ratio of relative intensities of the two molecular ion peaks of methyl bromide (CH3Br) in the mass spectrum is
- A disaccharide that will not give Benedict's test and will not form osazone is
- Choose the allowed transition


 Whatsapp
Whatsapp
 Facebook
Facebook



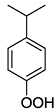 .
.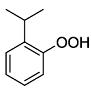 .
.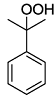 .
.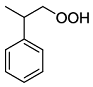 .
.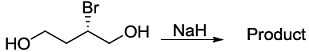
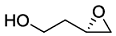 .
.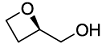 .
.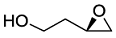 .
.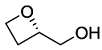 .
.