GATE 2017-2018 :: GATE Chemistry
- T he IUPAC nomenclature of Na[PCl6] is
- An intermediate formed during the hydroformylation of olefins using Co2(CO)8 as catalyst is
- The order of polarity of NH3, NF3 and BF3 is
- From a carboxymethyl-cellulose column at pH 6.0, arginine, valine and glutamic acid will elute in the order
- Symmetry operations of the four C2 axes perpendicular to the principal axis belong to the same class in the point group(s)
-
At 298 K, the EMF of the cell Pt|H2(1 bar)|H+(solution)||CI-|Hg2CI2|Hgis 0.7530 V. The standard potential of the calomel electrode is 0.2802 V. If the liquid junction potential is zero, the pH of the solution is
-
The wave function of a 1-D harmonic oscillator between x = +∞ and x = -∞ is given by Ψ(x) = N(2x2 - 1)e-x2/2. The value of N that normalizes the function Ψ(x) is
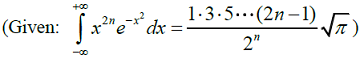
-
Consider the reactionH2 + C2H4 → C2H6The molecular diameters of H2 and C2H4 are 1.8 Å and 3.6 Å respectively. The pre-exponential factor in the rate constant calculated using collision theory in m3 (mole)-1 s-1 is approximately(For this reaction at 300 K, [(8kbT)/πμ]1/2 NA = 1.11 * 1027 m(mole)-1 s-1, where the symbols have their usual meanings)
-
The molecular partition function of a system is given by q(T) = [(kbT)/hc]3/2 [(8Ï€3mkbT)/h2]3/2, where the symbols have their usual meanings.The heat capacity at constant volume for this system is
-
Consider the phase diagram given below.
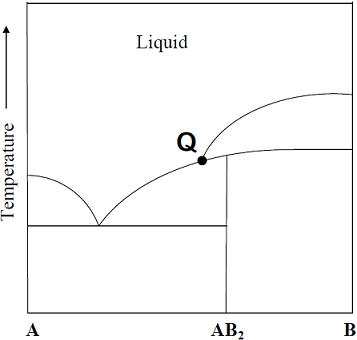 At the intersection point Q the phases that are in equilibrium are
At the intersection point Q the phases that are in equilibrium are


 Whatsapp
Whatsapp
 Facebook
Facebook

