GATE 2017-2018 :: GATE Chemistry
- The angular part of the wavefunction for the electron in a hydrogen atom is proportional to sin2θ cos θ e2iØ . The values of the azimuthal quantum number (l) and the magnetic quantum number (m) are, respectively
- T he bond that gives the most intense band in the infrared spectrum for its stretching vibration is
- I f xA and xB are the respective mole fractions of A and B in an ideal solution of the two and TA, TB, T are the fusion temperatures of pure A, pure B and the ideal solution respectively, then
-
F or a reaction involving two steps given below
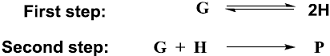 assume that the first step attains equilibrium rapidly. The rate of formation of P is proportional to
assume that the first step attains equilibrium rapidly. The rate of formation of P is proportional to - A metal chelate that can be used for separation and quantitative analysis of aluminium ions by gas chromatography is
- The enthalpies of hydration of Ca2+, Mn2+ and Zn2+ follow the order
- Among the following substituted silanes, the one that gives cross-linked silicone polymer upon hydrolysis is
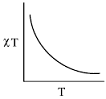
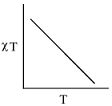
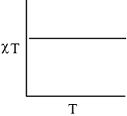
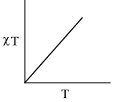
- The plot of χT versus T ((where χ is molar magnetic susceptibility and T is the temperature) for a paramagnetic complex which strictly follows Curie equation is
- A mong the following donors, the one that forms most stable adduct with the Lewis acid B(CH3)3 is


 Whatsapp
Whatsapp
 Facebook
Facebook


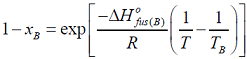 .
.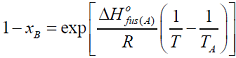 .
.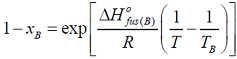 .
.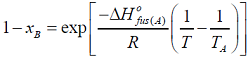 .
. .
. .
. .
. .
.