GATE 2017-2018 :: GATE Chemical
- In a 1-1 pass floating head type shell and tube heat exchanger, the tubes (od = 25 mm; id = 21 mm) are arranged in a square pitch. The tube pitch is 32 mm. The thermal conductivity of the shell side fluid is 0.19 W/m.K, and the Nusselt number is 200. The shell-side heat transfer coefficient (in W/m2. K), rounded off to the nearest integer, is
-
Match the process in Group I with the catalyst in Group II Group I Group IIP. Fischer-Tropsch synthesis I. NickelQ. Formaldehyde from methanol II. Fe2O3R. Hydrogenation of vegetable oils III. SilverS. Dehydrogenation of ethylbenzene IV. Cobalt
-
Match the polymer in Group I to the polymer characteristic in Group IIGroup I Group IIP. Polyethylene I. ElastomerQ. Phenol-formaldehyde polymer II. FiberR. Polyisoprene III. ThermoplasticS. Polyester IV. Thermosetting polymer
-
A counter-current extraction column is designed to remove 99% of solute C from a solution of solvent A and solute C using pure solvent B. The initial concentration of solute in the solution of A + C is 20 wt %, and the total flow of solution is 1000 kg/h. If the equilibrium relationship is Y = 2X , where Y = mass of C/mass of A and X = mass of C/mass of B.T he minimum flow rate of solvent B required (in kg/h) is
-
A counter-current extraction column is designed to remove 99% of solute C from a solution of solvent A and solute C using pure solvent B. The initial concentration of solute in the solution of A + C is 20 wt %, and the total flow of solution is 1000 kg/h. If the equilibrium relationship is Y = 2X , where Y = mass of C/mass of A and X = mass of C/mass of B. I f the flow rate of B is 2400 kg/h, then the theoretical number of stages in the column, using Kremser's equation (adjusted to the next integer) is
-
The reaction A(liq) + B(gas) ---> C(liq) + D(gas), is carried out in a reactor followed by a separator as shown below
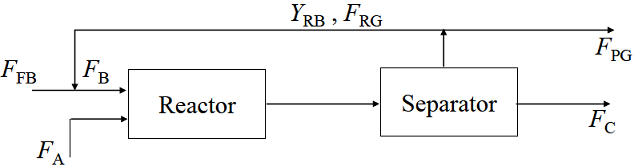 Notation:Molar flow rate of fresh B is FFBMolar flow rate of A is FAMolar flow rate of recycle gas is FRGMole fraction of B in recycle gas is YRBMolar flow rate of purge gas is FPGMolar flow rate of C is FCHere, FFB = 2 mol/s; FA = 1 mol/s, FB/FA = 5 and A is completely converted. If YRB = 0.3, the ratio of recycle gas to purge gas (FRG/FPG) is
Notation:Molar flow rate of fresh B is FFBMolar flow rate of A is FAMolar flow rate of recycle gas is FRGMole fraction of B in recycle gas is YRBMolar flow rate of purge gas is FPGMolar flow rate of C is FCHere, FFB = 2 mol/s; FA = 1 mol/s, FB/FA = 5 and A is completely converted. If YRB = 0.3, the ratio of recycle gas to purge gas (FRG/FPG) is -
The reaction A(liq) + B(gas) ---> C(liq) + D(gas), is carried out in a reactor followed by a separator as shown below
 Notation:Molar flow rate of fresh B is FFBMolar flow rate of A is FAMolar flow rate of recycle gas is FRGMole fraction of B in recycle gas is YRBMolar flow rate of purge gas is FPGMolar flow rate of C is FCHere, FFB = 2 mol/s; FA = 1 mol/s, FB/FA = 5 and A is completely converted.I f the ratio of recycle gas to purge gas (FRG/FPG) is 4 then YRB is
Notation:Molar flow rate of fresh B is FFBMolar flow rate of A is FAMolar flow rate of recycle gas is FRGMole fraction of B in recycle gas is YRBMolar flow rate of purge gas is FPGMolar flow rate of C is FCHere, FFB = 2 mol/s; FA = 1 mol/s, FB/FA = 5 and A is completely converted.I f the ratio of recycle gas to purge gas (FRG/FPG) is 4 then YRB is -
A Newtonian fluid of viscosity μ flows between two parallel plates due to the motion of the bottom plate (as shown below), which is moved with a velocity V . The top plate is stationary.
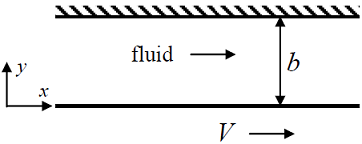 The steady, laminar velocity profile in the x - direction is
The steady, laminar velocity profile in the x - direction is -
A Newtonian fluid of viscosity μ flows between two parallel plates due to the motion of the bottom plate (as shown below), which is moved with a velocity V . The top plate is stationary.
 T he force per unit area (in the x -direction) that must be exerted on the bottom plate to maintain the flow is
T he force per unit area (in the x -direction) that must be exerted on the bottom plate to maintain the flow is -
The first order liquid phase reaction A ---> P is conducted isothermally in a plug flow reactor of 5 liter volume. The inlet volumetric flow rate is 1 liter/min and the inlet concentration of A is 2 mole/liter. If the exit concentration of A is 0.5 mole /liter, then the rate constant, in min-1, is


 Whatsapp
Whatsapp
 Facebook
Facebook

