GATE 2017-2018 :: GATE Chemical
- In a counter-flow double pipe heat exchanger, oil (m = 2 kg/s, CP = 2.1 kJ/kg.°C) is cooled from 90 °C to 40 °C by water (m = 1 kg/s, CP 4.2 kJ/kg.°C) which enters the inner tube at 10 °C. The radius of the inner tube is 3 cm and its length is 5 m. Neglecting the wall resistance, the overall heat transfer coefficient based on the inner radius, in kW / m2. K, is
- The rate-controlling step for the solid-catalyzed irreversible reaction A + B ---> C is known to be the reaction of adsorbed A with adsorbed B to give adsorbed C . If Pi is the partial pressure of component i and Ki is the adsorption equilibrium constant of component i , then the form of the Langmuir-Hinshelwood rate expression will be
-
Consider the drying operation shown in the figure below for a solid loading (dry basis) of 50 kg/m2 with a constant drying rate of 5 kg/m2.h. The falling rate of drying is linear with moisture content.
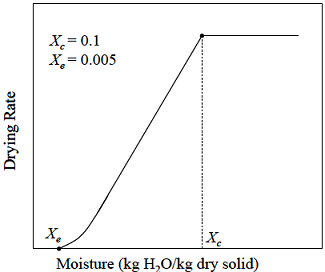 The drying time (in hrs) required to reduce an initial moisture content of 25% to a final moisture content of 2% is
The drying time (in hrs) required to reduce an initial moisture content of 25% to a final moisture content of 2% is - An equimolar mixture of A and B (A being more volatile) is flash distilled continuously at a feed rate of 100 kmol/h, such that the liquid product contains 40 mol % of A. If the relative volatility is 6, then the vapor product, in kmol/h, is
- A thermocouple having a linear relationship between 0oC and 350oC shows an emf of zero and 30.5 mV, respectively at these two temperatures. If the cold junction temperature is shifted from 0oC to 30oC, then the emf correction (in mV) is
- The characteristic equation for a system is s3 + 9s2 + 26s + 12(2 + Kc) = 0. Using the Routh test, the value of Kc that will keep the system on the verge of instability is
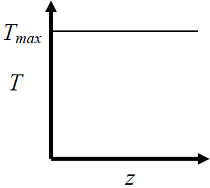
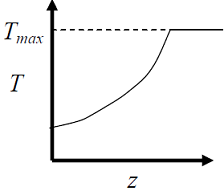
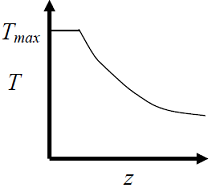
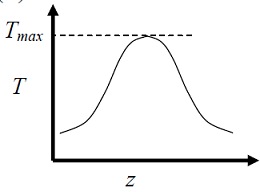
- The elementary reversible exothermic gas-phase reaction A + 3B <----> 2C, is to be conducted in a non-isothermal, non-adiabatic plug flow reactor. The maximum allowable reactor temperature is Tmax To minimize the total reactor volume, the variation of reactor temperature ( T ) with axial distance from the inlet ( ≈ ) should be
-
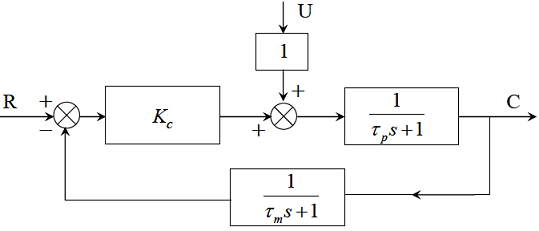
The block diagram of a system with a proportional controller is shown belowA unit step input is introduced in the set point. The value of Kc to provide a critically damped response for U = 0, Tp = 8 and Tm = 1 is
- A batch reactor produces 1 * 105 kg of a product per year. The total batch time (in hours) of the reactor is k√PB, where PB is the product per batch in kg and k = 1.0 h/√kg The operating cost of the reactor is Rs. 200/h. The total annual fixed charges are Rs. 340 * PB and the annual raw material cost is Rs. 2 * 106 The optimum size (in kg) of each batch (adjusted to the nearest integer) is
- Heat integration is planned in a process plant at an investment Rs. 2 * 106. This would result in a net energy savings of 20 GJ per year. If the nominal rate of interest is 15% and the plant life is 3 years, then the breakeven cost of energy, in Rs. per GJ (adjusted to the nearest hundred), is


 Whatsapp
Whatsapp
 Facebook
Facebook


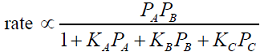 .
.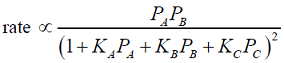 .
.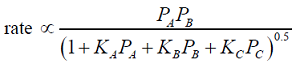 .
.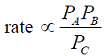 .
. .
. .
. .
. .
.