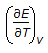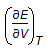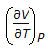Chemical Engineering :: Chemical Engineering Thermodynamics
-
For an isothermal process, the internal energy of a gas
-
In the equation, PVn = constant, if the value of n = 1, then it represents a reversible __________ process.
-
For the gaseous phase chemical reaction, C2H4(g) + H2O(g)
 C2H5OH(g), the equilibrium conversion does not depend on the
C2H5OH(g), the equilibrium conversion does not depend on the -
The first law of thermodynamics is a statement of conservation of
-
Which of the following non-flow reversible compression processes require maximum work ?
-
Pick out the correct statement.
-
"When a system in equilibrium is subjected to a change in temperature, pressure or concentration, the equilibrium is displaced in a direction which tends to undo the effect of the change." This is called the
-
Sound waves propagation in air exemplifies an __________ process.


 Whatsapp
Whatsapp
 Facebook
Facebook