Chemical Engineering :: Chemical Engineering Thermodynamics
-
The heat capacities for the ideal gas state depend upon the
-
Which of the following will increase the volume of a real gas by four times ?
-
Pick out the wrong statement.
-
The equation, PV = nRT, is best obeyed by gases at
-
Which of the following liquid metals has the highest thermal conductivity ?
-
Which of the following has the least thermal efficiency ?
-
If the internal energy of an ideal gas decreases by the same amount as the work done by the system, then the
-
Pick out the wrong statement.
-
Number of components (C), phase (P) and degrees of freedom (F) are related by Gibbs phase rule as
-
The value of Cp & Cv respectively for monoatomic gases in Kcal/kg Mole . °K are
|
A.
A refriferation cycle violates the second law of thermadynamics.
|
|
B.
Refrigeration cycle is normally represented by a temperature vs. entropy plot.
|
|
C.
In a refrigerator, work required decreases as the temperature of the refrigerator and the temperature at which heat is rejected increases.
|
|
D.
One ton of refrigeration is equivalent to the rate of heat absorption equal to 3.53 kW.
|
|
A.
The values of
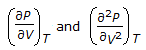 are zero for a real gas at its critical point. are zero for a real gas at its critical point. |
|
B.
Heat transferred is equal to the change in the enthalpy of the system, for a constant pressure, non-flow, mechanically reversible process.
|
|
C.
Thermal efficiency of a Carnot engine depends upon the properties of the working fluid besides the source & sink temperatures.
|
|
D.
During a reversible adiabatic process, the entropy of a substance remains constant.
|


 Whatsapp
Whatsapp
 Facebook
Facebook

