Chemical Engineering :: Chemical Engineering Thermodynamics
-
The enthalpy change when ammonia gas is dissolved in water is called the heat of
-
Adiabatic compression of a saturated water vapour makes it
-
For a multicomponent system, the term chemical potential is equivalent to the
-
The change in Gibbs freee energy for vaporisation of a pure substance is
-
Critical compressibility factor for all substances
-
For a given substance at a specified temperature, activity is __________ to fugacity.
-
In case of vapour compression refrigeration system, elevating the evaporator temperature (keeping the condenser temperature constant) results in
-
For water at 300°C, it has a vapour pressure 8592.7 kPa and fugacity 6738.9 kPa Under these conditions, one mole of water in liquid phase has a volume of 25.28 cm3 and that in vapour phase in 391.1 cm3.Fugacity of water (in kPa) at 9000 kPa will be
-
__________ equation predicts the activity co-efficient from experimental data.
-
As the temperature is lowered towards the absolute zero, the value of the quantity
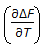 approaches
approaches


 Whatsapp
Whatsapp
 Facebook
Facebook

