GATE 2017-2018 :: GATE Metallurgical
- In a production facility, iron rods are made with a mean diameter of 6 cm and standard deviation of 0.02 cm. If a large number of rods are tested, the approximate percentage of rods whose sizes fall in the range of 5.98 cm to 6.02 cm is
- Which one of the following methods is NOT used for numerical integration?
-
How many boundary conditions are required to solve the following equation?
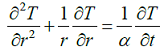
- When a zinc metal rod is immersed in dilute hydrochloric acid, it results in
- A fluid is flowing with a velocity of 0.5 m/s on a plate moving with a velocity of 0.01 m/s in the same direction. The velocity at the interface of the fluid and plate is
- Hot metal at 1700 K is poured in a sand mould that is open at the top. Heat loss from the liquid metal takes place by
- Which one of the following is an equilibrium defect?
- Floatation beneficiation is based on the principle of


 Whatsapp
Whatsapp
 Facebook
Facebook

