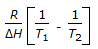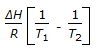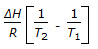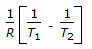Chemical Engineering :: Chemical Engineering Thermodynamics
-
 is the mathematical expression for
is the mathematical expression for -
In case of a close thermodynamic system, there is __________ across the boundaries.
-
As the temperature is lowered towards the absolute zero, the value of
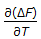 , then approaches
, then approaches -
Which of the following is not affected by temperature changes ?
-
For an ideal gas, the activity co-efficient is
-
Those solutions in which there is no volume change upon mixing the components in the liquid state and which, when diluted do not undergo any heat change (i.e. heat of dilution is zero), are called __________ solutions.
-
Generation of heat by friction is an example of a/an __________ change.
-
The extensive properties are


 Whatsapp
Whatsapp
 Facebook
Facebook