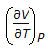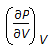Chemical Engineering :: Chemical Engineering Thermodynamics
-
The work done in an adiabatic change in a particular gas depends upon changes in the __________ only.
-
The value of gas constant 'R' is
-
In an isothermal process on an ideal gas, the pressure increases by 0.5 percent. The volume decreases by about __________ percent.
-
Which of the following is not a unit of the equilibrium constant Kp? (where, Δx = number of moles of products number of moles of reactants)
-
Pick out the correct statement.
-
Partial molal quantities are important in the study of
-
Critical solution temperature (or the con-solute temperature) for partially miscible liquids (e.g., phenol-water) is the minimum temperature at which
-
One ton of refrigeration is defined as the heat rate corresponding to melting of one ton of ice in one
|
A.
The available energy in an isolated system for all irreversible (real) processes decreases.
|
|
B.
The efficiency of a Carnot engine increases, if the sink temperature is decreased.
|
|
C.
The reversible work for compression in non-flow process under isothermal condition is the change in Helmholtz free energy.
|
|
D.
all (a), (b) and (c)
|


 Whatsapp
Whatsapp
 Facebook
Facebook