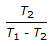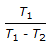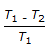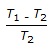Chemical Engineering :: Chemical Engineering Thermodynamics
-
Entropy change of mixing two liquid substances depends upon the
-
The four properties of a system viz. P, V, T, S are related by __________ equation.
-
If the pressure on 100 c.c. of air is halved, then its volume (at the same temperature) would be __________ c.c.
-
Pick out the wrong statement.
-
The principle applied in liquefaction of gases is
-
Co-efficient of performance for a reversed Carnot cycle working between temperatures T1 and T2 (T1 > T2) is
-
The number of degrees of freedom for an azeotropic mixture in a two component vapour-liquid equilibria is/are
-
Any substance above its critical temperature exists as
-
Which of the following decreases with increase in pressure ?
-
Isentropic process means a constant __________ process.
|
A.
Cp of monoatomic gases such as metallic vapor is about 5 kcal/kg.atom.
|
|
B.
The heat capacity of solid inorganic substance is exactly equal to the heat capacity of the substance in the molten state.
|
|
C.
There is an increase in entropy, when a spontaneous change occurs in an isolated system.
|
|
D.
At absolute zero temperature, the heat capacity for many pure crystalline substances is zero.
|


 Whatsapp
Whatsapp
 Facebook
Facebook