Chemical Engineering :: Stoichiometry
-
The viscosity of water at room temperature may be around one
-
A flowsheet is given in the following figure:
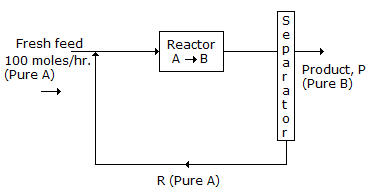
If the single pass once through conversion of A to B is 20%, then the rate of recycle R (molds/hr) is -
The heat capacity of most substances is greater for the __________ state.
-
The temperature at which real gases obey the ideal gas law over a wide range of pressure is called the __________ temperature.
-
Elements in a periodic table are arranged in order of their
-
For most salts, the solubility increases with rise in temperature, but the solubility of __________ is nearly independent of temperature rise.
-
The molecules of a liquid which is in equilibrium with its vapor at its boiling point on an average have equal __________ in the two phases.
-
If a solution of eutectic composition is cooled, __________ reaching the eutectic temperature.
-
The density of a gas 'X' is twice that of another gas 'Y'. If the molecular weight of gas 'Y' is 'M'; then the molecular weight of the gas 'X' will be


 Whatsapp
Whatsapp
 Facebook
Facebook

