Chemical Engineering :: Chemical Process
-
In multistage equilibrium conversion of SO2 to SO3 (2SO2 + O2
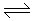 2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by
2SO3), the reverse reaction becomes appreciable at a temperature of 550° C. The percentage equilibrium conversion of SO2 to SO3 can be increased by -
Tall oil obtained as a by-product from the black liquor recovery is
-
Styrene is produced from ethyl benzene by the process of
-
Acetone is produced by catalytic dehydrogenation of
-
Argon is the third largest constituent of air (followed by N2 & O2). Its percentage by volume in air is
-
Bakelite is


 Whatsapp
Whatsapp
 Facebook
Facebook

