Chemical Engineering :: Stoichiometry
-
Internal energy is independent of the __________ for an ideal gas.
-
__________ fuels require the maximum percentage of 'excess air' for complete combustion.
-
Pick out the wrong statement:
-
__________ equation relates latent heat and boiling point.
-
At higher temperature, molal heat capacities of most of the gases (at constant pressure) __________ with increase in temperature.
-
The heat evolved in the combustion of benzene is represented by the equation:
C6H6 + 7.5 O2 = 6CO2 + 3H2O, ΔH = 3264.6 kJ/kg. mole
The heat energy change, when 39 gm of C6H6 is burnt in an open container, will be __________ kJ/kgmole. -
Pick out the wrong statement about the recycle stream in a process.
-
An aqueous solution of 2.45% by weight H2SO4 has a specific gravity of 1.011. The composition expressed in normality is
-
Which of the following ideal gas laws are not applicable to mixture of gases ?
|
A.
Clausius-Clapeyron equation relates the latent heat of vaporisation to the slope of the vapor pressure curve.
|
|
B.
At the boiling point of liquid at the prevailing total pressure, saturated absolute humidity is infinite.
|
|
C.
Percentage saturation and relative saturation are numerically equal for an unsaturated vapor gas mixture.
|
|
D.
Clapeyron equation is given by,
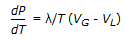 ; where, P = vapor pressure, T = absolute temperature, λ = latent heat of vaporisation, VG and VL = volumes of gas and liquid respectively. ; where, P = vapor pressure, T = absolute temperature, λ = latent heat of vaporisation, VG and VL = volumes of gas and liquid respectively. |
|
A.
Recycling in a process stream helps in utilising the valuable reactants to the maximum with minimum loss of reactants.
|
|
B.
The ratio of the quantity of a reactant present in the reactor feed of a recycling operation to the quantity of the same reactant entering the process as fresh feed is called combined feed ratio.
|
|
C.
Recycling in a process does not help in getting higher extent of reaction.
|
|
D.
Recycling is exemplified by refluxing back a part of the distillate to the distillation column to maintain the quantity of liquid within the column.
|


 Whatsapp
Whatsapp
 Facebook
Facebook

