Discussion :: Stoichiometry
-
Pick out the correct statement.
|
A.
Heat of solution is always positive.
|
|
B.
At equilibrium, ΔG is zero.
|
|
C.
For the reaction, PCl5
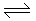 PCl3 + Cl2, ΔG is less than ΔE PCl3 + Cl2, ΔG is less than ΔE |
|
D.
The heating of water in a beaker is an example of an isolated system.
|
Answer : Option B
Explanation :
No answer description available for this question.
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

