Chemical Engineering :: Stoichiometry
-
The reaction A + B → C has been conducted in a reactor as shown below.
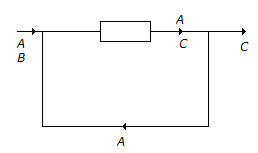
The number of balances (material) that can be made around the reactor are -
The number of water molecules present in a drop of water weighing 0.018 gm is 6.023 x __________
-
The density of a liquid is 1500 kg/m3. Its value in gin/litre will be equal to
-
The heat of vaporisation __________ with increase in pressure.
-
Which of the following expressions defines the Baume gravity scale for liquids lighter than water ?
-
Which of the following is followed by an ideal solution ?
-
One of the specific gravity scales is "Brix" (used speicifically for sugar solution). It is defined as
-
Pick out the wrong statement.
|
A.
Ten times dilution of a normal solution (N) reduces its normality to N/10.
|
|
B.
When equal weights of oxygen and methane are mixed in an empty reactor at room temperature, then the fraction of total pressure exerted by the oxygen is 1/2.
|
|
C.
Volume occupied by 9.034 x 1023 atoms of oxygen in ozone (O3) at NTP will be 11200 c.c
|
|
D.
One kg mole of an ideal gas at N.T.P occupies 22400 Nm3.
|


 Whatsapp
Whatsapp
 Facebook
Facebook

