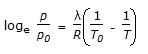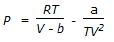Chemical Engineering :: Stoichiometry
-
With rise in temperature, the heat capacity of a substance
-
Which of the following is the Claussius-Clayperon equation ?
-
Kopp's rule is useful for the determination of
-
Pick out the wrong unit conversion.
-
Viscosity of 1 centipoise is equal to 1 centis-toke in case of


 Whatsapp
Whatsapp
 Facebook
Facebook