Chemical Engineering :: Stoichiometry
-
During a phase change process like sublimation, vaporisation, melting etc., the specific __________ does not change.
-
The boiling points for pure water and pure toluene are 100°C and 110.6°C respectively. Toluene and water are completely immiscible in each other. A well agitated equimolar mixture of toluene and water are prepared. If, at a total pressure of one standard atm. exerted by the vapours of water and toluene, the mole fraction of water Xw in the vapour phase satisfies
-
The heat of adsorption of a gas caused by Van der Walls forces of attraction and capillarity is equal to the heat of
-
The pressure of 'V' litres of a dry gas is increased from 1 to 2 kgf/cm2 at a constant temperature. The new volume will become
-
At standard conditions,
N2 + 2O2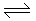 2NO2; ΔG° = 100 kJ/mole
2NO2; ΔG° = 100 kJ/mole
NO +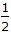 O2
O2 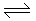 2NO2; ΔG° = -35 kJ/mole
2NO2; ΔG° = -35 kJ/mole
The standard free energy of formation of NO in kJ/mole is -
1 kg of calcium carbide (CaC2) produces about 0.41 kg of acetylene gas on treatment with water. How many hours of service can be derived from 1 kg of calcium carbide in an acetylene lamp burning 35 litres of gas at NTP per hour ?


 Whatsapp
Whatsapp
 Facebook
Facebook

