Chemical Engineering :: Nuclear Power Engineering
-
Spent fuel from the nuclear thermal reactor contains
-
A boiling water reactor is the one, in which the
-
Which is used as a coolant in nuclear reactor due to its high capture cross-section ?
-
Moderating material used in a thermal-reactor should be a
-
Ordinary water is not used as a moderator because, it
-
The atomic number of a radioactive element is not changed, when it emits __________ rays.
-
Fission of U-235 on slow neutron bombardment can be represented by
-
A nuclear reactor can't be used for
-
Which of the following is a moderating material used in nuclear reactor ?
-
The atomic number of an element is equal to the number of __________ present in its atom.


 Whatsapp
Whatsapp
 Facebook
Facebook


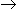 56Ba143 + 36Kr90
56Ba143 + 36Kr90