Discussion :: Chemical Reaction Engineering
-
The rate equation for the reaction represented by,
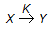 is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
is given by - rx = K1 . Cx/(1 + K2 Cx). At high value of Cx (i.e.., K2Cx > > 1), the order of the reaction and the rate constant are respectively
Answer : Option A
Explanation :
No answer description available for this question.
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

