Discussion :: Chemical Reaction Engineering
-
A batch adiabatic reactor at an initial temperature of 373°K is being used for the reaction, A
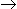 B. Assume the heat of reaction is - 1kJ/mole at 373°K and heat capacity of both A and B to be constant and equal to 50J/mole.K. The temperature rise after a conversion of 0.5 will be
B. Assume the heat of reaction is - 1kJ/mole at 373°K and heat capacity of both A and B to be constant and equal to 50J/mole.K. The temperature rise after a conversion of 0.5 will be
Answer : Option B
Explanation :
No answer description available for this question.
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

