Discussion :: Chemistry
- The ionisation energy of hydrogen atom in the ground state is x KJ. The energy required for an electron to jump from 2nd orbit to 3rd orbit is
Answer : Option A
Explanation :
The ionisation energy for the hydrogen atom can be written as:
Ionisation energy = energy in first orbit - energy in infinite orbit
Ionisation energy = E = -E0 / n2 , where E0 = 13.6 eV (1 eV = 1.602×10-19 Joules) and n = 1,2,3… and so on.
The difference in energy = Einfinity - E1
so the ionisation energy from the ground state = x KJ = 0-( -E0 /12 ) = E0
The energy required to jump the elctron from second to the third stage is: 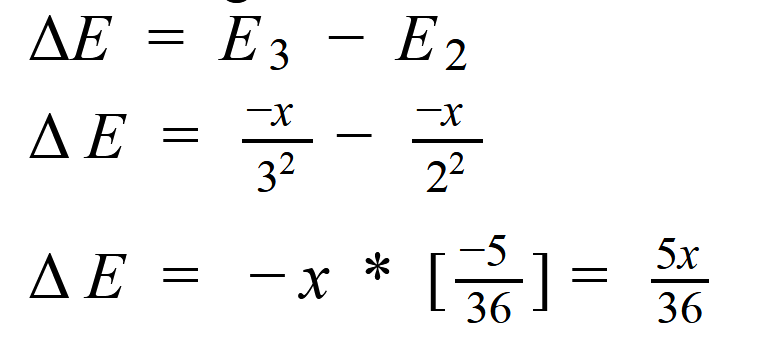
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

